In this judgment at the High Court, Lord Justice Arnold stepped down from the Court of Appeal to the High Court to hear the case. His findings on infringement may be an indication of Arnold’s desire to set some practical limits on the first and third Actavis equivalence questions. In particular, he applies a rigorous evidential approach to the determination of whether the variant achieves substantially the same result in substantially the same way. His decision will be welcomed by practitioners looking for clarity on when a variant may not infringe under the UK equivalence test.
Background
The long running Actavis vs Eli Lilly battle finally ended in the UK in 2017 when the Supreme Court found that Actavis’ products infringed Eli Lilly’s patent. Summaries of this landmark judgement can be found here.
A significant outcome of the Supreme Court’s judgement was a new and broader test to assess infringement by the doctrine of equivalence.
The equivalence test applies when the allegedly infringing product or process falls outside of the literal (or normal/purposive) meaning of a claim. The court then determines whether the alleged infringing product or process (the “variant”) nevertheless infringes the patent through equivalence as an immaterial variant.
The UK equivalence test takes the form of the following three questions:
(i) Notwithstanding that it is not within the literal meaning of the relevant claim(s) of the patent, does the variant achieve substantially the same result in substantially the same way as the invention, i.e. the inventive concept revealed by the patent?
(ii) Would it be obvious to the person skilled in the art, reading the patent at the priority date, but knowing that the variant achieves substantially the same result as the invention, that it does so in substantially the same way as the invention?
(iii) Would such a reader of the patent have concluded that the patentee nonetheless intended that strict compliance with the literal meaning of the relevant claim(s) of the patent was an essential requirement of the invention?
Infringement by equivalence occurs if answering yes, yes, no to the questions.
This test introduced a much lower threshold for a finding of equivalence than the previous UK test, seemingly tipping the balance toward patentees. The intention it is argued was to bring UK jurisprudence closer to that of our European counterparts. However, its critics argue that unlike the law in, for example, Germany, where the application of equivalence is limited in practice by case law, for example in chemical and mathematical range cases, the UK law has no such body of limiting case law.
As well as lowering the threshold for equivalence, the Supreme Court’s Actavis v Eli Lilly judgement was also ground-breaking because it found equivalence for chemical subject matter. The judgement essentially found that a potassium counter ion was equivalent to a sodium counter ion. The lowering of the threshold for equivalence applied to chemical subject matter led to some concern in the Life Science and Chemical sectors where it is generally possible, and often necessary, to use precise claim language such that failure to literally claim the infringing subject matter can generally be regarded as intentional and a strict requirement of the claim.
Decision
In the High Court decision between Akebia Therapeutics and Fibrogen Inc., Lord Justice Arnold found five of the six patents invalid for insufficiency and then applied the equivalence test to the sixth patent when considering if the compound vadadustat infringed Claim 17A (Compound C) of EP(UK)2,289,531.
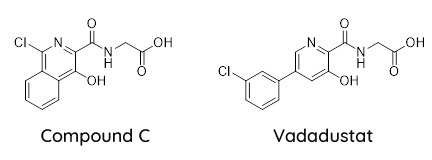
To a non-chemist, it may seem that these two compounds are somewhat similar. However, in vadadustat the chlorine atom (“Cl”) is now found on a pendant phenyl ring substituent and not on an isoquinoline ring. In fact, vadadustat does not have an isoquinoline ring.
The judge found that the inventive concept is a matter of interpretation and whether the variant achieves the same result in the same way is partly a question of fact and that therefore the burden of proof for this test lies with the patentee.
On the question of interpretation, Arnold found that the inventive concept was not any compound that treats renal anaemia by inhibiting HIF-PH as argued by the claimant but the use of the specific compound C (a specific molecule) for treating renal anaemia by inhibiting HIF-PH. On this basis, to determine whether the variant achieved substantially the same result in substantially the same way it was necessary for Lord Arnold to consider the structure of the respective molecules and their mechanisms of action. By taking a narrow interpretation of the inventive concept, Lord Arnold has arguably raised the bar for the patentee considerably because proving that two molecules behave in the same way can be very difficult in Life Sciences.
Upon consideration of the available facts, Lord Arnold was persuaded inter alia, that there was a likelihood that the binding and specificity of vadadustat was not the same as Compound C; that the vadadustat inhibition via competition with 2-OG was still unknown for Compound C; and that relying on a similarity in structure (an asserted “common structural motif”) was not persuasive because asserted points of similarity were not claimed or mentioned in the description and the structural motif could not exclude the isoquinoline ring system.
As such, the patentee was unable to provide sufficient evidence to prove that vadadustat worked in the same way (or even substantially the same way) as the inventive concept.
In summary, it appears that the bar of evidence required to prove equivalence with such complex biochemical subject matter is perhaps a higher one than could previously have been inferred from the Supreme Court’s Actavis judgement.
Despite finding no infringement based on an assessment of question (i) alone, Lord Justice Arnold went on to consider the further Actavis equivalence questions (ii) and (iii).
Lord Justice Arnold comments with regard to question (ii) that due to the way the question is formulated, if question (i) is answered “yes” then question (ii) will almost always be answered “yes”, as it would be in this case – “there will rarely be scope for a negative answer if the answer to question (i) is “yes””.
Regarding question (iii), the judge found that it was clear that the patentee intended that strict compliance with the normal meaning of “Compound C” was an essential requirement of the invention of claim 17A for which he listed eight reasons which are summarised below.
- Claim 17A is limited by structure to Compound C alone. There is no mention in claim 17A to any broader structure.
- Read in the context of the specification, claim 17A is clearly intended to be narrow because Formula (I) defines a broader scope, followed by Formula (Ia) to (Id) which is narrower than Formula (I) and broader than claim 17A.
- Compound C is the most promising and best-explored compound and so a technical choice has been made in limiting to claim 17A.
- Granted claim 1 has been amended down to claim 17A and other granted claims have been deleted. The skilled team is therefore aware that broader claims are likely to be invalid and therefore, not intended.
“By amending down to Compound C, the Claimants are disclaiming the other ways of achieving the same effect disclosed in the specification, and in particular everything covered by the broader granted claims.”
- Vadadustat is less structurally similar to Compound C than Compounds D to K. The patentee has disclaimed Compounds D to K and therefore does not intend that a claim to Compound C would extend to cover vadadustat, “an instance of a principle recognised by the BGH … see Case X ZR 69/10 – Diglyzidverbindung (Diglycid compound) at [45]-[46]”.
- The scope of claim 17A by equivalence according to a broad approach to question (i) would extend to compounds shown to be HIF-PHIs in the “Epstein” prior art document. Since this would result in an invalid claim due to lack of obviousness, therefore, the scope of claim 17A would not be intended to extend beyond Compound C.
- Additionally, such a broad scope of claim 17A by equivalence would result in problems with insufficiency due to both plausibility and undue burden. Therefore, this similarly supports that the scope is not intended to extend beyond Compound C.
- Lord Justice Arnold referencing Actavis v Lilly states this is one of the cases where it would be contrary to the public interest for the contents of the prosecution file to be ignored.
In the prosecution history, claim 1 was limited in scope to compounds of Formula (I) to overcome a novelty objection by the examiner. Fibrogen had represented that it was not seeking to contend that the scope extends to heterocyclic carboxamides beyond Formula (I). However, the judge found that that was precisely what they were trying to do by presenting that the claim extends to this surrendered subject matter due to equivalence.
Of the eight reasons given, it seems that some at least could also have been applied in the Supreme Court’s Actavis judgement, such as reasons 1 to 4. However, in this case, the reason for limiting the claims by amendment did not include added matter but was one of invalidity due to prior art.
Interestingly, the fifth reason follows German case law in that limiting the claims effectively disclaims the other disclosed compounds meant that less structurally similar compounds to the disclaimed compounds could not infringe. In Actavis, the potassium salt was not disclosed in the patent in question so Lord Justice Arnold has every right to apply this test. It could provide much needed clarity in the future following claim restrictions during examination.
Perhaps the most interesting of Lord Justice Arnold’s reasons is reason 8 which opens the door to prosecution history review on the grounds of public interest. In this case, the patentee represented a contrary view to the examiner to that which it now relied on. In addition, he points out that the patentee could have appealed to the European Patent Office’s Board of Appeal for broader claims and did not do so. He ends his discussion in view of this, by pointing out that extending the scope of protection of claim 17A in the manner contended for by the Claimants would go well beyond fair protection for the patentee and would not afford a reasonable degree of legal certainty for third parties.
This decision provides useful tests to apply to the law of equivalence questions especially when applied to different chemical molecules in the area of Life Sciences for the consideration of the Actavis equivalence questions. It remains to be seen whether this approach will be adopted by the higher courts.
Comment: –
It is difficult to escape the conclusion that Lord Justice Arnold does not sit easily with all the findings of the Actavis decision. In this judgement, he seems to find practical ways to limit its effect. The case provides useful guidance on many issues including:- on the effect of claim limitations, at least those based on validity, on later equivalence arguments; on the necessity for the patentee to provide strong and persuasive evidence that the alleged infringement provides the same result as the invention and in the same way; on claims limited to molecules likely forming part of the invention and to argue a broader inventive concept free of structural limitations may ultimately be unsuccessful; and on statements made by the attorney in prosecution not being in the public interest to ignore in legal proceedings asserting the contrary.